11 Mar 2021
FDA clearance for the OCULUS Pentacam® AXL Wave with optical biometry, retroillumination, objective refraction and wavefront aberrometry
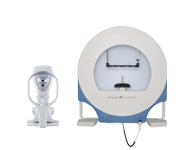
The new OCULUS Pentacam® AXL Wave has received the 510(k) clearance of the US Food and Drug Administration (FDA). The Pentacam® AXL Wave represents the continued development of the successful and time-proven Pentacam® portfolio. The established functions of anterior segment tomography and optical biometry have been complemented by retroillumination and a Hartman-Shack sensor for total eye wavefront and objective refraction.
The Pentacam® AXL Wave is the latest and most versatile member of the Pentacam® family, incorporating intuitive reports based on clinical studies, with guaranteed network compatibility and full operability of all Pentacam® software and examinations.
A new examination routine guides the user through the imaging process. The measuring process is straightforward, user-independent, fast and patient-friendly. The intuitive guide allows both eyes to be examined in less than 5 minutes.
These new hardware and software features allow optical performance analysis of the total cornea, total eye and crystalline lens or implanted IOL. In addition, the new Pentacam® AXL Wave comes with a comprehensive, clearly arranged overview display showing all parameters for corneal screening, IOL power selection, IOL power calculation, ICL selection and calculation and pupil diameter under dimmed and dark conditions.
The standard software package with the IOL calculator and its built-in IOL database provide verification of findings in numerous clinical applications such as corneal refractive surgery, lens refractive surgery, presbyopia solutions and standard cataract surgery.
Please contact your local distributor for more information. www.pentacam.com/axl-wave
About OCULUS Optikgeräte GmbH
OCULUS Optikgeräte GmbH is a family-run business located in Wetzlar, Germany. Since 1895, OCULUS has been a partner for eye care professionals around the world. With about 245 employees, OCULUS consistently develops and produces an extensive range of over 150 products at their headquarters in Wetzlar. All of OCULUS's products are manufactured exclusively in Germany, with over 50% of production distributed worldwide.
Back to: Press Information